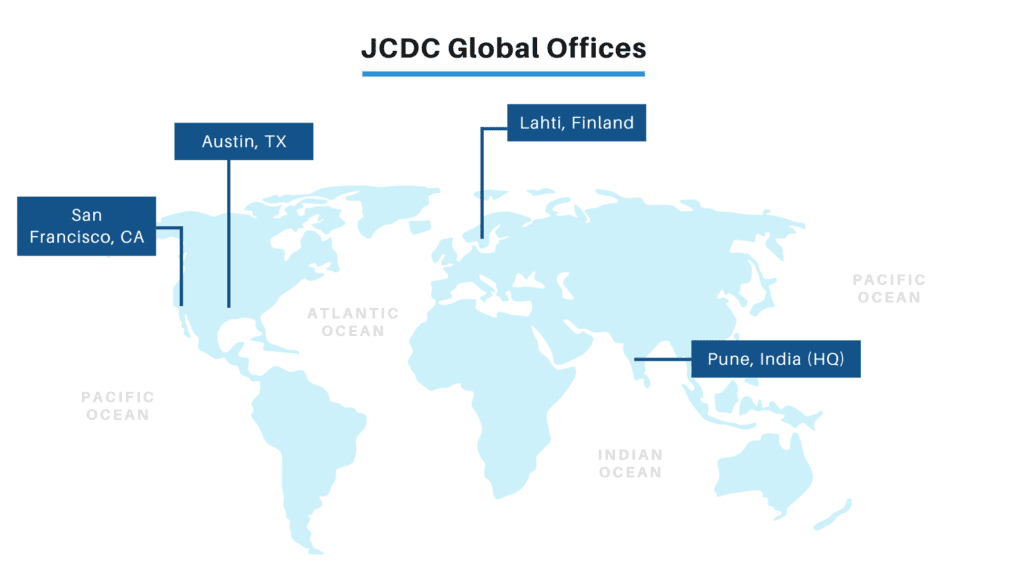
ABOUT
JCDC
ABOUT
JCDC
JCDC is a fast growing, global, clinical-stage full service CRO. We provide end-to-end services for Phase I-IV Clinical Trials, Validation Studies, Real World Evidence (RWE) Studies and Biobanking Projects. Till date we have successfully executed 350+ clinical projects.
JCDC was formed in 2006 as a managed research centre within Jehangir Hospital, Pune. JCDC is one of the few research centres in India to be AAHRPP accredited and ISO 9001:2008 certified. JCDC has also been awarded as the “ Best Investigator Site” by the Indian Society of Clinical Research (ISCR) in 2012.
JCDC’s expertise is in Medical Writing & Publications, Monitoring and Project Management, Clinical Data Management, Biostatistics and Site Management.
What makes us unique is our digital transformation and early adoption of decentralized clinical trials, which have allowed us to conduct clinical trials with minimum manual/paper processes and reduced the need for physical travel to sites. This results in significant cost and time savings for our clients.
Our multi-disciplinary team (including clinicians, researchers, regulatory and operational experts), hands-on approach, flexibility and complete transparency allows us to offer you customized solutions and study designs for your clinical trial’s success.
At JCDC, we try and maintain the highest level of ethics, integrity and transparency in our interactions with patients and in our relationships with our customers. Our focus is on offering premium quality services and our goal is to strive for excellence.
In essence, JCDC stands for Clinical Precision
Our Vision
reliable organization in the field of
clinical research in India.
Our Mission
patient care and furthering the cause of global research.
We will achieve this by taking immense joy in our work and
providing premium quality services to our customers.
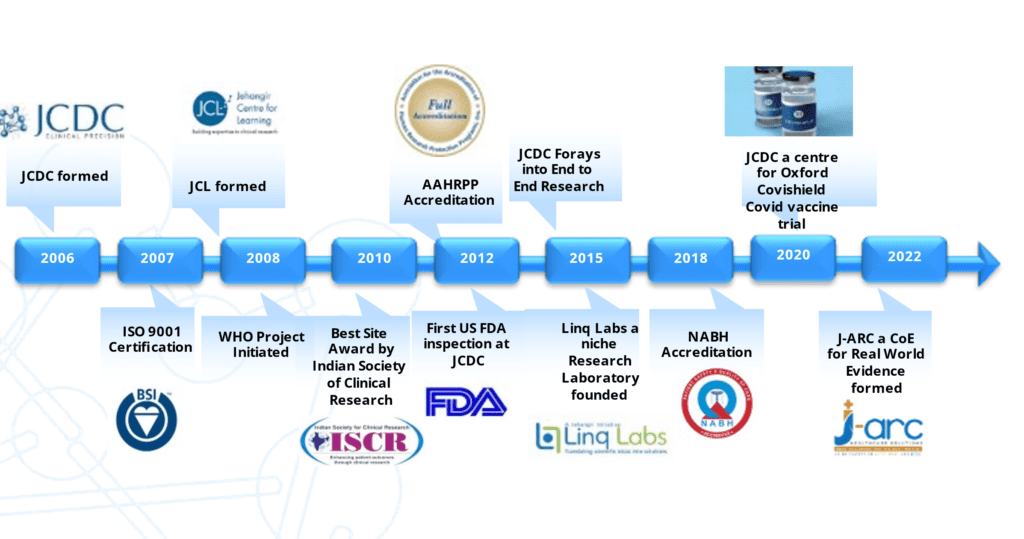

JCDC Reformed
2006

]CL formed
2008

AAHRPP Accreditation
2012
JCDC Forays into End to End Research
2015
 JCDC a centre for Oxford Covishield Covid vaccine trial
JCDC a centre for Oxford Covishield Covid vaccine trial2020
 JARC a CoE for Real World Evidence formed
JARC a CoE for Real World Evidence formed
2022
2006
2007
2008
2010
2012
2015
2018
2020
2022
ISO 9001 Certification

2008
WHO Project Initiated
Best Site Award by Indian Society of Clinical Research

First US FDA inspection at ]CDC

Ling Labs a niche Research Laboratory founded

NABH Accreditation

ISO 9001 Certification

2008
WHO Project Initiated
Best Site Award by Indian Society of Clinical Research

First US FDA inspection at ]CDC

Ling Labs a niche Research Laboratory founded

NABH Accreditation

We place the safety and concern for our patients at the forefront of all our decisions and actions. We take individual and collective responsibility for their well-being.
Our focus is on driving quality and our goal is to strive for excellence. We learn from our mistakes by understanding the root cause and devise effective and realistic solutions
We listen carefully to the ‘Voice of the customer’ to understand them better in order to provide better service each time and exceed their expectations
Our business must make a sound profit to provide for adverse times & allow for continued investment in the future to provide more opportunities to employees.
We encourage open and honest communication in all our relationships to resolve issues, exchange information and share knowledge
We have mutually beneficial partnerships with suppliers giving them an opportunity to make a fair profit
All our actions, decisions and policies exemplify our honesty, ethics and integrity
We work as a family. We respect the dignity and recognize the merit of our employees. We provide opportunities to employees and are mindful of ways to help our employees fulfill their family responsibilities. Employees feel free to make suggestions and complaints.