FULL SERVICE CRO
JCDC is a fast growing, global, clinical-stage full service CRO. We provide end-to-end services for Phase I-IV clinical trials, Validation studies, Real World Evidence (RWE) Studies or Biobanking projects.
JCDC expertise is in Medical Writing, Monitoring and Project Management, Clinical Data Management, Biostatistics and Site Management.
What makes us unique is our digital transformation and early adoption of decentralized clinical trials, which have allowed us to conduct clinical trials with minimum manual/paper processes and reduced the need for physical travel to sites. This results in significant cost and time savings for our clients.
Our multi-disciplinary team (including clinicians, researchers, regulatory and operational experts), hands-on approach, flexibility and complete transparency allows us to offer you customized solutions and study designs for your clinical trial’s success.




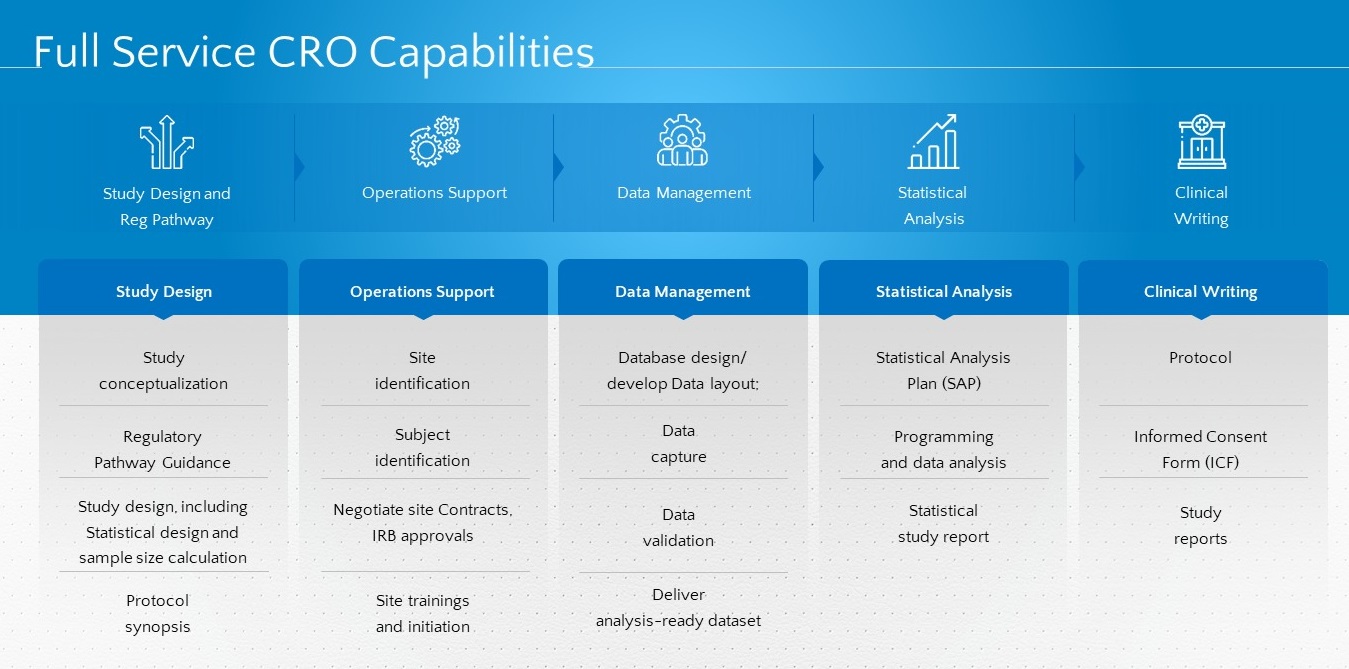
Study
Conceptualization
Regulatory
Pathway Guidance
Study design, including statistical design and sample size calculation
Protocol
synopsis
Site
identification
Subject
identification
Negotiate
site Contracts, IRB
approvals
Site trainings and initiation
Database design/ develop Data layout:
Data
capture
Data
validation
Deliver analysis-ready dataset
Statistical Analysis Plan (SAP)
Programming and data analysis
Statistical study report
Protocol
Informed Consent Form (ICF)
Study reports

Study Design and
Reg Pathway

Study Design and
Reg Pathway

Study Design and
Reg Pathway

Study Design and
Reg Pathway

Study Design and
Reg Pathway
Study Design
Study Design
Study Design
Study Design
Study Design
Regulatory Pathway Guidance
Study Conceptualization
Regulatory Pathway Guidance
Regulatory Pathway Guidance
Study Conceptualization
Regulatory Pathway Guidance
Regulatory Pathway Guidance
Study Conceptualization
Regulatory Pathway Guidance
Regulatory Pathway Guidance
Study Conceptualization
Regulatory Pathway Guidance
Regulatory Pathway Guidance
Study Conceptualization
Regulatory Pathway Guidance





Writing
services
Consent Form,
Study reports. Literature
review and
Meta-analysis
Based on years
of Clinical
Operations experience
at the Site level
Management and Biostatistics services
from Study startup, study conduct and Close Out
Availability of 21 CFR Part 11 compliant EDC platforms
Regulatory Consulting including regulatory pathway to US FDA, EMEA and MHRA.
Central Ethics committee
services
- Focus Areas as a CRO
Phase 1 – 4 Clinical Trials - Real World Evidence Studies
- Post Marketing Surveillance (PMS) Studies
- Observational / Registry Studies
- Pilot and Pivotal Validation Studies
- Safety & Efficacy Testing Services for Medical Devices
- Claims Support Studies Safety & Efficacy Testing Services Ffor Cosmetics, Dermaceutical, OTC and Phyto-Pharmaceutical Products
- Nutrition Studies Glycemic Index, Growth& immunity, Bone Health, Cognitive Functions, Obesity, Metabolic disorders, Gastrointestinal Disorders etc
