SITE
MANAGEMENT
SITE
MANAGEMENT
Our expertise is in over 20 major therapeutic areas

A separate dedicated clinical research facility within the hospital premises complements the work of the investigators by providing them vital administrative and infrastructural support. This is the JCDC model.
This has made us a distinctive network of investigator sites allowing us to gain the trust all top ten pharmaceutical companies and most of the large international Contract Research Organizations worldwide.
- JCDC has built a highly interactive database of 300+ sites and 3,500 Investigators across India covering all major therapeutic areas
- We have conducted a survey/enquiry from each of the sites on 52 data points. Further, our interactive tool can drill down the site/ investigator details using single or multiple combinations
- This database can expedite site identification after which study-specific site feasibility and site qualification visit
(SQV) can be conducted
The scope of our service include
Our project management and study coordinators provide support to the investigators in speedy recruitment of patients. A database of thousands of patients admitted in the hospital and those availing out-patient facilities across therapeutic area is maintained to aid in recruitment. This ensures that patients from various ethnic backgrounds and demographics are considered for the trial.
Our full time staff of Project Managers, Quality Assurance managers and study co-ordinators ensure that planning and execution of the clinical trials is smoothly managed from start to finish. Our experienced project management makes every effort to deliver results error free and within the specified time frame.
We effectively address all the training needs of our investigators by organizing ongoing and refresher in ICH GCP, Schedule Y and trainings required for effectively conducting a trial. Besides, we organize outside training on a regular basis to keep both our staff and investigators abreast of all latest trends especially in technology in order to hone their proficiency.
Site Selection Process
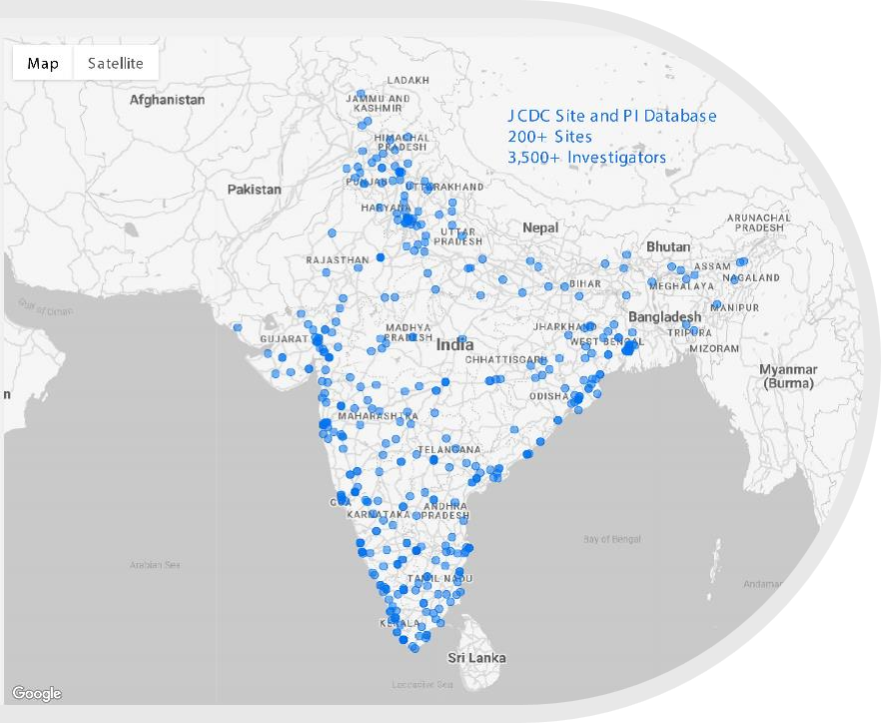
Database of over 200+ sites and
3,500 investigators in India

Our database for each site covers 52 data points, including the studies done in the past, Indication & Phase of study. infrastructure at site etc.

Our sites are across India and cover all major
therapeutic areas

Once the study is kicked off, we identify the initial sites from our database and conduct study-specific site feasibilities to filter the top sites

Site Qualification Visits are then done for the selected sites to arrive at the final sites for Sponsor approval

Database of over 200+ sites and
4,000 investigators in India

Our database for each site covers 52 data points, including the studies done in the past, Indication & Phase of study. infrastructure at site etc.

Our sites are across India and cover all major
therapeutic areas

Once the study is kicked off, we identify the initial sites from our database and conduct study-specific site feasibilities to filter the top sites

Site Qualification Visits are then done for the selected sites to arrive at the final sites for Sponsor approval
- JCDC has built a highly interactive database of 200+ sites and 3,500 Investigators across India covering all major therapeutic areas
- We have conducted a survey/enquiry from each of the sites on 52 data points. Further, our interactive tool can drill down the site/ investigator details using single or multiple combinations
- This database can expedite site identification after which study-specific site feasibility and site qualification visit
(SQV) can be conducted
